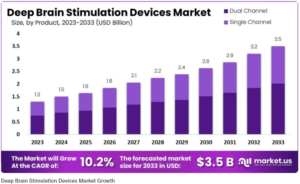
A recent paper published by market.us projects that the Deep Brain Stimulation (DBS) devices market is expected to reach around $3.5 Billion by 2033, compared to the projected $1.5 Billion for 2024.
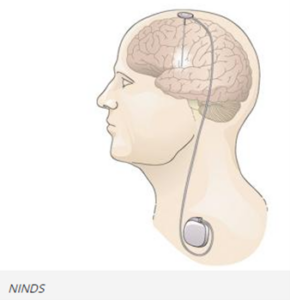
DBS systems can be used for treatment of Parkinson’s disease and other movement disorders. They can include a device that sends electrical signals through wire electrodes implanted in the brain, similar to how a cardiac pacemaker sends electrical signals to the heart. DBS systems interrupt the irregular signals that cause tremors and other movement symptoms when they are successfully implemented. Doctors have used DBS systems to treat movement disorders or neuropsychiatric conditions when medications have become less effective.
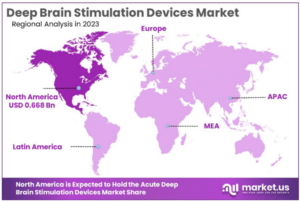
According to market.us, the growing number of neurological disorders such as Parkinsons’s disease, essential tremor, dystonia, and epilepsy, is a significant factor driving the growth of the DBS devices industry. Furthermore, the increasing demand for minimally invasive surgical procedures augmented with growing rate of geriatric population have also been identified as some of the most crucial drivers for deep brain simulators market growth. And while there is a global DBS devices market, North America is expected to remain the market leader with a market share exceeding 51.4% in 2023.
Recently, multiple companies have made announcements related to the DBS devices market. For example, on January, 25, 2024, Abbott Laboratories announced that it has received FDA approval for its Liberta Rechargeable (RC) DBS system as an Implantable Pulse Generator (IPG). According to Abbott, Liberta stands to be the world’s smallest rechargeable DBS IPG with a volume that is nearly a third smaller than other DBS devices currently available in the U.S. Abbott also says the Liberta RC DBS system has the longest-lasting battery charge of any FDA-approved DBS system. It can also receive and adjust therapy remotely in conjunction with Abbott’s NeuroSphere Virtual Clinic, which can allow patients to communicate with their doctors via video chat, and their doctors can adjust the neurostimulation system’s setting remotely. Additionally, Medtronic announced just weeks ago that it secured FDA approval for its Percept™ RC DBS system, which according to Medtronic, is the thinnest dual channel neurostimulator DBS system on the market.
