Medical device companies are often well versed in prosecuting multiple patents within a single “family.” Indeed, such families are often of significant value to early stage (and later stage) medical device companies. But a Federal Circuit decision from last year raised the possibility that the continued prosecution of patents within a family could invalidate those early, valuable patents. In a clarifying decision, the Federal Circuit recently explained that “a first-filed, first-issued, later-expiring claim (e.g., in a Parent Patent) cannot be invalidated (under the doctrine of obviousness-type double patenting – “ODP”) by a later-filed, later-issued, earlier-expiring reference claim (e.g., in a child patent) having a common priority date.” This may sound complicated, but it can be good news for patent owners who own a family of patents because it is now less likely that a later prosecution will invalidate an earlier issued patent.
Allergan USA v. MSN Labs, provides clarity to a specific fact pattern common in the medical device and pharmaceutical industries. This case applies to patent families like those shown in the figure below. A parent application is filed (orange dot) and issued (green dot) after delays in prosecution. One or more child applications (e.g., continuations or divisionals) are filed, claiming priority to the parent. The children claim patentably indistinct subject matter relative to the Parent. The children are filed after and issue after the parent, with less delay. To compensate for delays during the prosecution of the parent patent, the parent received a patent term adjustment (PTA), pushing back the expiration date (red dot) of the parent by, for example, 467 days. The children patents received less PTA (in this example, the children received no PTA). As a result, the parent expires after the children. Allergan clarifies that in this scenario, the child cannot invalidate the parent for ODP.
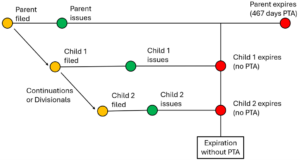
Before Allergan, there was confusion and concern among patent owners. A 2023 Federal Circuit decision, In re Cellect LLC, included language suggesting that a parent patent with PTA could be invalid over the earlier-expiring child patent claiming patentably indistinct subject matter, under ODP. For example, an upcoming IPWatchDog event includes a panel session titled “Patent Term Adjustment after In re Cellect.” The abstract to the panel session hints at this confusion, reciting that “[r]ecent judicial holdings (e.g., In re Cellect) have significantly weakened PTA and have in some cases created ‘traps’ which subvert congressional intent.”
In Allergan, the Federal Circuit noted that “[ODP’s] primary goal is to prevent an unjustified timewise extension of patent exclusivity beyond the life of a patent.” The (now-reversed) lower court in Allergan followed this logic, citing Cellect: after the Children expire, the claimed subject matter (and obvious variants thereof) should belong to the public. Since the Parent patent purports to claim exclusivity of an obvious variant of the Children’s claimed subject matter for another 400-plus days, the Parent patent must be invalid under ODP as seeking an unjustified timewise extension of patent exclusivity. The Federal Circuit reversed the lower court’s decision, deciding that the Cellect conclusion does not govern the case shown in the figure above. Instead, the Federal Circuit concluded “that the claims of the (Children patents) are not proper ODP references that can be used to invalidate (Claims of the Parent patent).”
The Federal Circuit clarified that the purpose of the ODP doctrine “is to prevent patentees from obtaining a second patent on the patentably indistinct invention to effectively extend the life of a first patent to that subject matter.” The Federal Circuit concluded “that a first-filed, first-issued, later-expiring claim (e.g., in the Parent patent) cannot be invalidated by a later-filed, later-issued, earlier-expiring reference claim (e.g., in the Children patents) having a common priority date.” The Federal Circuit’s decision is available here.
