The FDA issued a warning letter to Abiomed, for failing to seek approval for its Impella Connect System software before putting it on the market. The Impella Connect System software is designed to work with the company’s Automated Impella Controller (AIC), which is part of a medical device system that provides temporary ventricular support to help a patient’s heart to pump blood in a critical care setting.
Abiomed attempted to argue that functions of the Impella Connect System were non-device clinical decision support (CDS) software functions or non-device medical device data systems, but the FDA disagreed. The FDA asserted that the software component, by providing “time-critical alarms and patient-specific medical information intended to trigger potential clinical intervention to assure patient safety,” qualifies as a medical device requiring premarket authorization. This warning letter highlights the FDA’s intention to enforce regulatory oversight of medical device data systems that go beyond basic electronic data transfer and storage functions. The development is significant for companies involved in wearable medical devices and patient monitoring technologies.
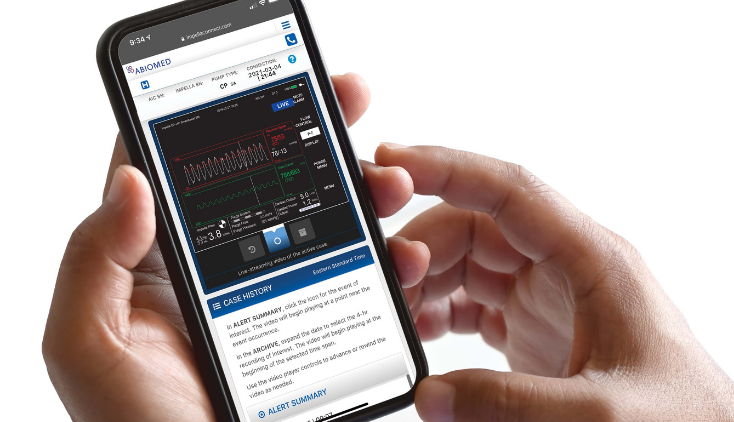
The warning letter is just one example of the FDA’s broader ongoing efforts to adapt to the challenges posed by digital health technologies including clinical decision support software, AI-enabled medical devices, digital therapeutics, wearables, remote patient monitoring, and more.
Notably, the FDA recently announced that it is establishing a Digital Health Advisory Committee. The committee, set to be fully operational in 2024, will consist of experts from various disciplines who will advise FDA leaders on digital health-related matters, with the goal of ensuring the safety and effectiveness of emerging technologies without stifling innovation. It will be accepting applications until December 11th, 2023.
